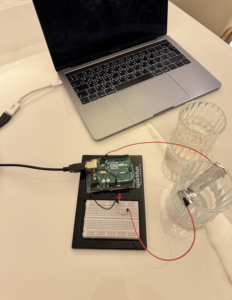
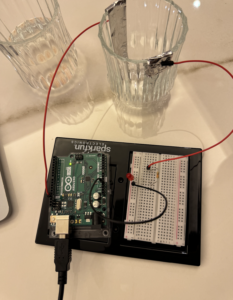
For this assignment, I setup a cup of saltwater with two wires connecting to two aluminum foils, which were positioned in an empty cup. Next, I configured the Arduino board, connecting an LED to the breadboard along with a resistor. Powering up the Arduino with the computer, we poured the saltwater into the empty cup. As the saltwater facilitated the flow of current between the electrodes, the circuit was completed, and the LED was activated.
Saltwater lights up the LED by serving as a conductive medium that allows the flow of electricity. When the salt dissolves in the water, it separates into positively and negatively charged ions, making the water conductive. As the two electrodes, placed in the saltwater, complete the circuit, the flow of current is facilitated through the saltwater. This flow of electricity activates the LED, causing it to emit light. The presence of the salt enables the completion of the electrical pathway, allowing the LED to be powered and produce illumination.
https://youtube.com/shorts/xvapKBzMCXU?feature=share
One practical application of the saltwater conductivity experiment could be in the field of emergency lighting systems. In remote or disaster-prone areas where access to conventional power sources is limited, this saltwater-based circuit could serve as an emergency lighting solution. For instance, in regions prone to natural disasters such as hurricanes or earthquakes, where power outages are common, individuals could use this simple saltwater-powered LED setup as an alternative lighting source. This cost-effective and simple solution could significantly contribute to enhancing safety and visibility in challenging circumstances where traditional power sources are unavailable.